Phenol oxidation by Fenton's reagent (H2O2 + Fe+2) in aqueous solution has been studied for the purpose of learning
more about the reactions involved and the extent of the oxidation process, under various operating conditions. An initial
phenol concentration of 100 mg/L was used as representative of a phenolic industrial wastewater. Working temperature
of 25C was tested, and initial pH was set at 5.6 . The H2O2 and the Fe+2 doses were varied in the range of
(H2O2/Fe+2/phenol = 3/0.25/1 to 5/0.5/1). Keeping the stirring speed of 200 rpm.
The results exhibit that the highest phenol conversion (100%) was obtained under (H2O/Fe+2/phenol ratio of 5/0.5/1)
at about 180 min. The study has indicated that Fenton's oxidation i
 (110)
(110)
 (100)
(100)
Heavy metal ion removal from industrial wastewater treatment systems is still difficult because it contains organic contaminants. In this study, functional composite hydrogels with photo Fenton reaction activity were used to decompose organic contaminants. Fe3O4 Nanoparticle, chitosan (CS), and other materials make up the hydrogel. There are different factors that affected Photo-Fenton activity including (pH, H2O2 conc., temp., and exposure period). Atomic force microscopy was used to examine the morphology of the composite and its average diameter (AFM). After 60 minutes of exposure to UV radiation, CS/ Fe3O4 hydrogel composite had degraded methylene blue (M.B.)
... Show More (4)
(4)
Titanium oxide nanoparticles-modified smectite (SMC-nTiO2) as a low-cost adsorbent was investigated for the removal of Rhodamine B (RhB) from aqueous solutions. The adsorbents (SMC and SMC-nTiO2) were characterized by scanning electron microscopy, Fourier transforms infrared spectroscopy, and energy-dispersive X-ray spectroscopy. The effects of various parameters like contact time, adsorbent weight, pH, and temperatures were examined. Three kinetic equations (pseudo-first-order (PFO), pseudo-second-order (PSO), and intra-particle diffusion) were used to evaluate the experimental kinetic of the data and the results showed that the adsorption process is in line with the PSO kinetic model. Adsorption equilibrium isotherms were modeled using La
... Show More (6)
(6)
 (3)
(3)
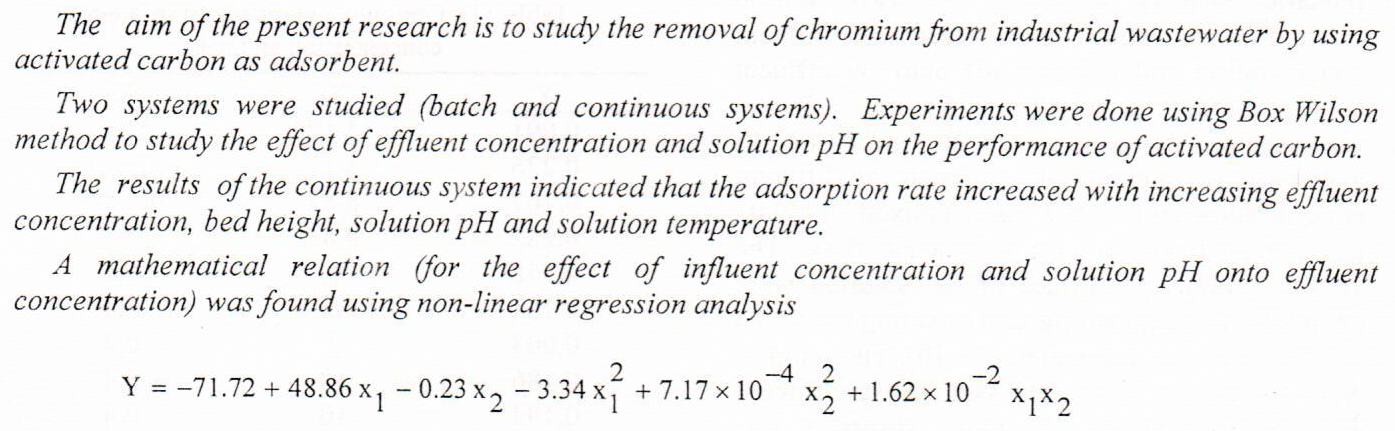
In this paper activated carbon adsorbents produced from waste tires by chemical activation methods and application of microwave assisted KOH activation. The influence of radiation time, radiation power, and impregnation ratio on the yield and oil removal which is one of the major environmental issues nowadays and considered persistent environmental contaminants and many of them are suspected of being carcinogenic. Based on Box-Wilson central composite design, polynomial models were developed to correlate the process variables to the two responses. From the analysis of variance the significant variables on each response were identified. Optimum conditions of 4 min radiation time, 700 W radiation power and 0.5 g/g impregnation ratio
... Show MorePhosphorus is usually the limiting nutrient for eutrophication in inland receiving waters; therefore, phosphorus concentrations must be controlled. In the present study, a series of jar test was conducted to evaluate the optimum pH, dosage and performance parameters for coagulants alum and calcium chloride. Phosphorus removal by alum was found to be highly pH dependent with an optimum pH of 5.7-6. At this pH an alum dosage of 80 mg/l removed 83 % of the total phosphorus. Better removal was achieved when the solution was buffered at pH = 6. Phosphorus removal was not affected by varying the slow mixing period; this is due to the fact that the reaction is relatively fast.
The dosage of calcium chloride and pH of solution play an importa
 (35)
(35)
 (24)
(24)
This work was conducted to study the treatment of industrial waste water, and more particularly those in the General Company of Electrical Industries.This waste water, has zinc ion with maximum concentration in solution of 90 ppm.
The reuse of such effluent can be made possible via appropriate treatments, such as chemical coagulation, Na2S is used as coagulant.
The parameters that influenced the waste water treatment are: temperature, pH, dose of coagulant and settling time.
It was found that the best condition for zinc removal, within the range of operation used ,were a temperature of 20C a pH value of 13 , a coagulant dose of 15 g Na2S /400ml solution and a settling time of 7 days. Under these conditions the zinc concentrat
