A nano manganese dioxide (MnO2) was electrodeposited galvanostatically onto a carbon fiber (CF) surface using the simple method of anodic electrodeposition. The composite electrode was characterized by field emission scanning electron microscopy (FESEM), and X-ray diffraction (XRD). Very few studies investigated the efficiency of this electrode for heavy metals removal, especially chromium. The electrosorption properties of the nano MnO2/CF electrode were examined by removing Cr(VI) ions from aqueous solutions. NaCl concentration, pH, and cell voltage were studied and optimized using the Box-Behnken design (BDD) to investigate their effects and interactions on the electrosorption process. The results showed that the
... Show More (3)
(3)
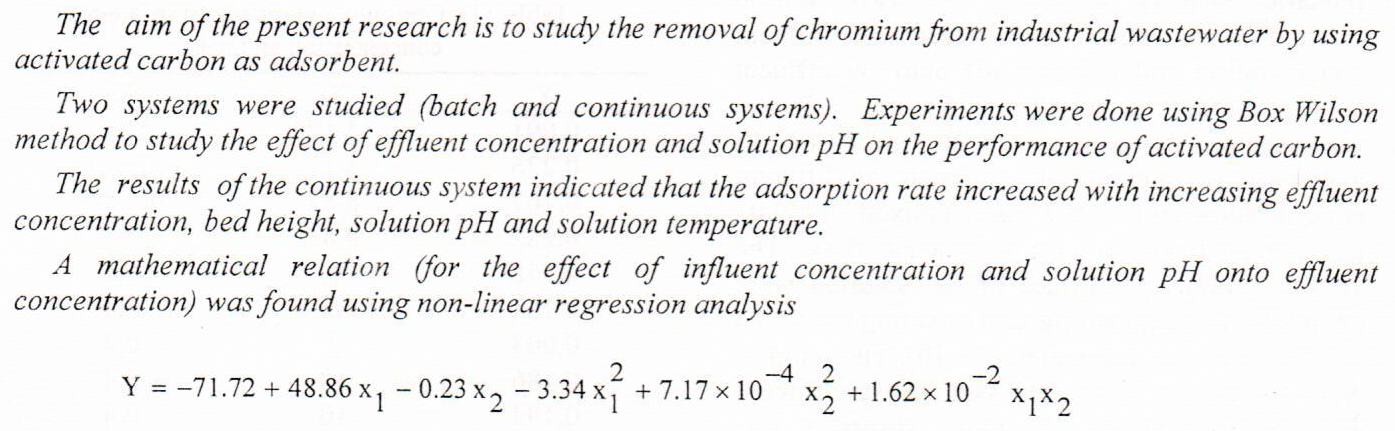
 (3)
(3)
In this study three reactive dyes (blue B, red R and yellow Y) in single , binary and ternary solution were adsorbed by activated carbon AC in equilibrium and kinetic experiments. Surface area, Bulk and real density, and porosity were carried out for the activated carbon.
Batch Experiments of pH (2.5-8.5) and initial concentration (5-100) mg/l were carried out for single solution for each dye. Experiments of adsorbent dosage effect (0.1-1)g per 100 ml were studied as a variable to evaluate uptake% and adsorption capacity for single dyes(5, 10) ppm, binary and ternary (10) ppm of mixture solutions solution of dyes. Langmuir, and Freundlich, models were used as Equilibrium isotherm models for single solution. Extended Langmuir and Freun
 (5)
(5)
In the present study, advanced oxidation process / heterogeneous photocatalytic process (UV/TiO2/Fenton) system was investigated to the treatment of oily wastewater. The present study was conducted to evaluate the effect of hydrogen peroxide concentration H2O2, initial amount of the iron catalyst Fe+2, pH, temperature, amount of TiO2 and the concentration of oil in the wastewater. The removal efficiency for the system UV/TiO2/Fenton at optimal conditions and dosage (H2O2 = 400mg/L, Fe+2 = 40mg/L, pH=5, temperature =30oC, TiO2=75mg/L) for 1000mg/L load was found to be 77%.
Aluminum foil cover around the re
... Show MoreAbstract This study investigated the treatment of textile wastewater contaminated with Acid Black 210 dye (AB210) using zinc oxide nanoparticles (ZnO NPs) through adsorption and photocatalytic techniques. ZnO NPs were synthesized using a green synthesis process involving eucalyptus leaves as reducing and capping agents. The synthesized ZnO NPs were characterized using UV-Vis spectroscopy, SEM, EDAX, XRD, BET, Zeta potential, and FTIR techniques. The BET analysis revealed a specific surface area and total pore volume of 26.318 m2/g. SEM images confirmed the crystalline and spherical nature of the particles, with a particle size of 73.4 nm. A photoreactor was designed to facilitate the photo-degradation process. The study investigated the inf
... Show More (11)
(11)
 (3)
(3)
Crude soybean peroxidase (SBP), isolated from soybean seed coats (hulls) at unusually low concentrations, catalyses the oxidative polymerisation of hazardous aqueous benzidine and its 3,3′-dichloro, 3,3′-dimethyl and 3,3′-dimethoxy derivatives in the presence of hydrogen peroxide. The optimum operating conditions for oxidation of 0·10 mM benzidine were investigated. At pH 5, the hydrogen peroxide-to-substrate concentration ratio was 1·5 and the minimum SBP concentration required to achieve at least 95% conversion of the benzidine in synthetic wastewater was 0·43 mU/ml. Progress curves were established for the conversion of the four substrates, and apparent first-order rate constants were derived. Enzyme-catalysed polym
... Show More (13)
(13)
 (10)
(10)
Single long spiral tube column pressure swing adsorption (PSA) unit, 25 mm diameter, and 6 m length was constructed to study the separation of water from ethanol at azeotropic concentration of 95 wt%. The first three meters of the column length acted as a vaporizer and the remaining length acted as an adsorber filled by commercial 3A zeolite. The effect of pressure, temperature and feed flow rate on the product ethanol purity, process recovery and productivity were studied. The results showed that ethanol purity increased with temperature and pressure and decreased with feed flow rate. The purity decreased with increasing productivity. The purity range was 98.9 % to 99.6 %, the recovery range was 0.82 to 0.92 and the productivity range w
... Show MoreIn this study, Yogurt was dried and milled, then shaked with distilled water to remove the soluble materials, then again dried and milled. Batch experiments were carried out to remove hexavalent chromium from aqueous solutions. Different parameters were optimized such as amount of adsorbent, treatment time, pH and concentration of adsorbate. The concentrations of Cr6+ in solutions are determined by UV-Visible spectrophotometer. Maximum percentage removal of Cr6+ was 82% at pH 2. Two equilibrium adsorption isotherms mechanisms are tested Langmuir and Freundlich, the results showed that the isotherm obeyed to Freundlich isotherm. Kinetic models were applied to the adsorption of Cr6+ ions on the adsorbents, ps
... Show More (3)
(3)
 (1)
(1)
The objective of this study is to investigate the application of advanced oxidation processes (AOPs) in the treatment of wastewater contaminated with furfural. The AOPs investigated is the homogeneous photo-Fenton (UV/H2O2/Fe+2) process. The experiments were conducted by using cylindrical stainless steel batch photo-reactor. The influence of different variables: initial concentration of H2O2 (300-1300mg/L), Fe+2(20-70mg/L), pH(2-7) and initial concentration of furfural (50-300 mg/L) and their relationship with the mineralization efficiency were studied.
Complete mineralization for the system UV/H2O2/Fe+2 was achieved at: initi
... Show MoreThe nanostructured Manganese dioxide/Carbon fiber (CF) composite electrode was prepared galvanostatically using a facile method of anodic electrodeposition by varying the reaction time and MnSO4 concentration of the electrochemical solution. The effects of these parameters on the structures and properties of the prepared electrode were evaluated. For determining the crystal characteristics, morphologies, and topographies of the deposited MnO2 films onto the surfaces of carbon fibers, the X-ray diffraction (XRD), field emission scanning electron microscopy (FESEM), and atomic force microscopy (AFM) techniques were used, respectively. It found that the carbon fibers were coated with γ-MnO2 with a density that increased with increasing the de
... Show More (3)
(3)
 (2)
(2)
