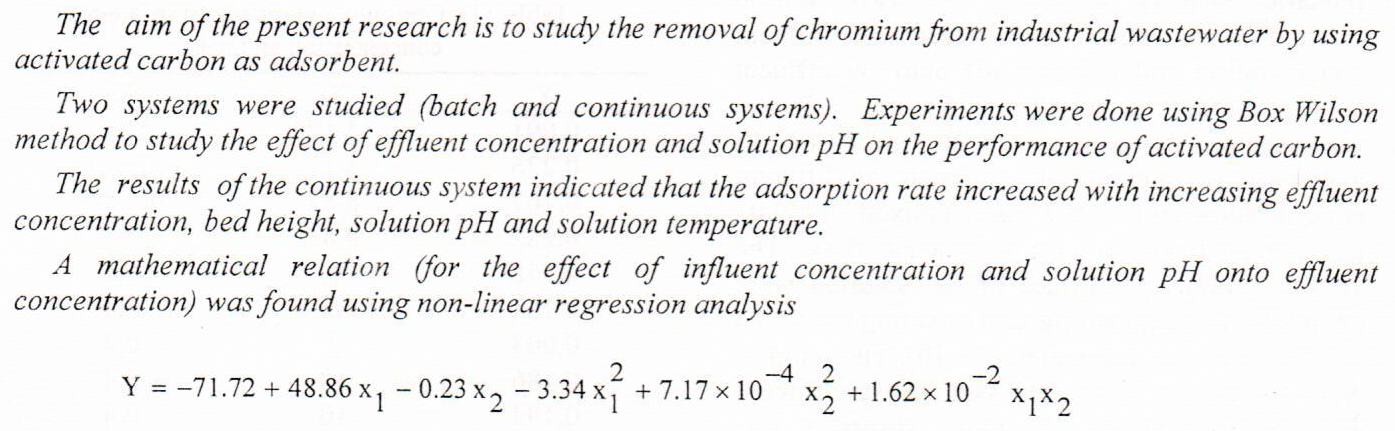
 (38)
(38)
In this research a local adsorbent was prepared from waste tires using two-step pyrolysis method. In the carbonization process, nitrogen gas flow rate was 0.2L/min at carbonization temperature of 500ºC for 1h. The char products were then preceded to the activation process at 850°C under carbon dioxide (CO2) activation flow rate of 0.6L/min for 3h. The activation method produced local adsorbent material with a surface area and total pore volume as high as 118.59m2 /g and 0.1467cm3/g, respectively. The produced . local adsorbent (activated carbon) was used for adsorption of lead from aqueous solution. The continuous fixed bed column experiments were conducted. The adsorption capacity performance of prepared activated carbons in this work
... Show More (22)
(22)
 (24)
(24)
Adsorption techniques are widely used to remove organics pollutants from waste water particularly, when using low cost adsorbent available in Iraq. Al-Khriet powder which was found in legs of Typha Domingensis is used as bio sorbent for removing phenolic compounds from aqueous solution. The influence of adsorbent dosage and contact time on removal percentage and adsorb ate amount of phenol and 4- nitro phenol onto Al-Khriet were studied. The highest adsorption capacity was for 4-nitrophenol 91.5% than for phenol 82% with 50 mg/L concentration, 0.5 gm. dosage of adsorbent and pH 6 under a batch condition. The experimental data were tested using different isotherm models. The results show that Freundlich model resulted in the best fit also
... Show MoreThe presence of heavy metals in the environment is major concern due to their toxicity. In the present study a strong acid cation exchange resin, Amberlite IR 120 was used for the removal of lead, zinc and copper from simulated wastewater. The optimum conditions were determined in a batch system of concentration 100 mg/L, pH range between 1 and 8, contact time between 5 and 120 minutes, and amount of adsorbent was from 0.05 to 0.45 g/100 ml. A constant stirring speed, 180 rpm, was chosen during all of the experiments. The optimum conditions were found to be pH of 4 for copper and lead and pH 6 for zinc, contact time of 60 min and 0.35 g of adsorbent. Three different temperatures (25, 40 and 60°C) were selected to investigate the effect
... Show More (7)
(7)
The research aims to use a new technology for industrial water concentrating that contains poisonous metals and recovery quantities from pure water. Therefore, the technology investigated is the forward osmosis process (FO). It is a new process that use membranes available commercial and this process distinguishes by its low cost compared to other process. Sodium chloride (NaCl) was used as draw solution to extract water from poisonous metals solution. The driving force in the FO process is provided by a different in osmotic pressure (concentration) across the membrane between the draw and poisonous metals solution sides. Experimental work was divided into three parts. The first part includes operating the forward osmosis process using T
... Show More (4)
(4)
Activated carbon prepared from date stones by chemical activation with ferric chloride (FAC) was used an adsorbent to remove phenolic compounds such as phenol (Ph) and p-nitro phenol (PNPh) from aqueous solutions. The influence of process variables represented by solution pH value (2-12), adsorbent to adsorbate weight ratio (0.2-1.8), and contact time (30-150 min) on removal percentage and adsorbed amount of Ph and PNPh onto FAC was studied. For PNPh adsorption,( 97.43 %) maximum removal percentage and (48.71 mg/g) adsorbed amount was achieved at (5) solution pH,( 1) adsorbent to adsorbate weight ratio, and (90 min) contact time. While for Ph adsorption, at (4) solution pH, (1.4) absorbent to adsorbate weight ratio, and (120 min) contact
... Show More (4)
(4)
Background: Large amounts of oily wastewater and its derivatives are discharged annually from several industries to the environment. Objective: The present study aims to investigate the ability to remove oil content and turbidity from real oily wastewater discharged from the wet oil's unit (West Qurna 1-Crude Oil Location/ Basra-Iraq) by using an innovated electrocoagulation reactor containing concentric aluminum tubes in a monopolar mode. Methods: The influences of the operational variables (current density (1.77-7.07 mA/cm2) and electrolysis time (10-40 min)) were studied using response surface methodology (RSM) and Minitab-17 statistical program. The agitation speed was taken as 200 rpm. Energy and electrodes consumption had been studi
... Show More (20)
(20)
 (63)
(63)
 (51)
(51)
